Clinically clean production for maximum quality and stability.
Systems for the manufacturing of pharmaceutical and medical products are subject to strict rules and standards. Good Manufacturing Practice (GMP) guidelines and Food and Drug Administration (FDA) regulations are two key examples. These requirements also apply to material supply systems – even where core components are located outside the clean room. motan provides a variety of solutions to meet these challenges.
We offer a broad portfolio of high-quality components for the pharmaceutical and medical sectors, designed and built to fulfil exceptional standards of cleanliness.
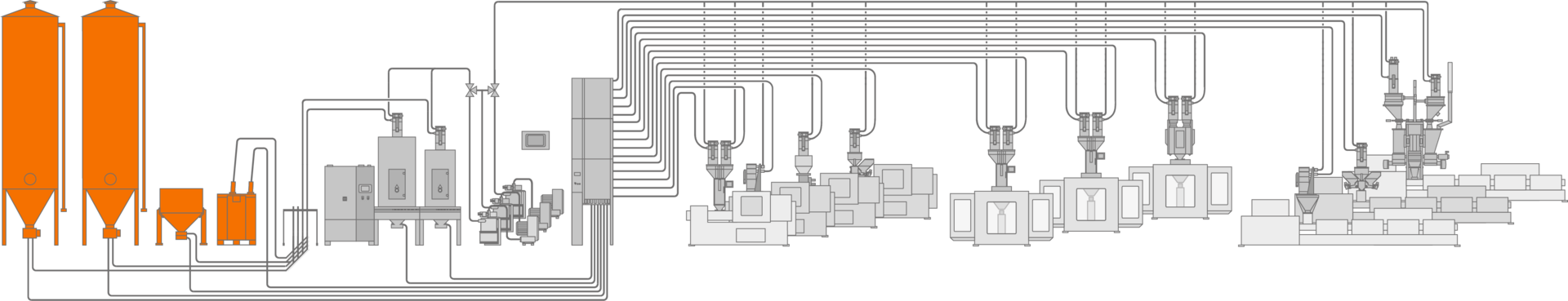
Contamination-free storage.
From small, portable containers to large outside silos, motan offers a wide range of storage solutions for contamination-free materials handling in line with your specific requirements

End-to-end documentation of drying parameters.
High-quality documentation comes as standard with our drying systems. The built in DryingOrganizer ensures excellent and reliable reproducible results, every time. And patented ETA plus® technology enables you to cut energy consumption by upt to 64 per cent.
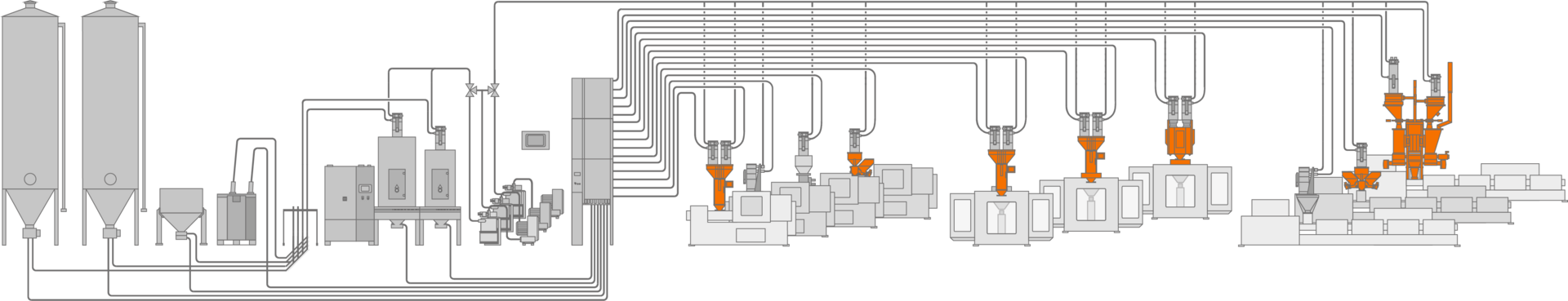
High reproducibility and recipe accuracy.
motan's gravimetric dosing systems dose ingredients with pinpoint accuracy and unbeatable consistency – the ideal basis for all forms of qualification.
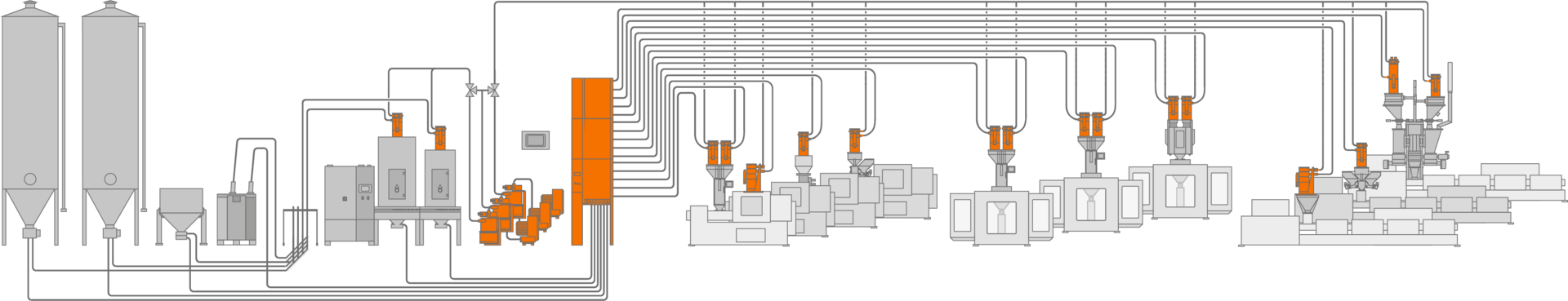
Material flow in line with your needs.
Custom-tailored, accurate, material-friendly, and contamination-free - motan's conveying systems, featuring Intelliflow®, centrally control, monitor, document, and optimize your material flow.
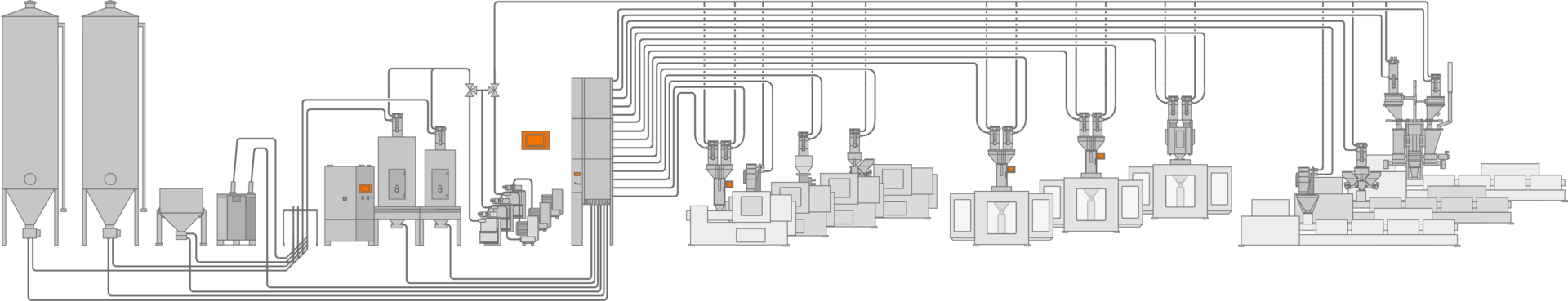
Intelligent control network.
Thanks to validation-ready software and end-to-end quality monitoring, the flow of materials is documented at all stages, from delivery to your plant to the final product.

A lasting partnership. Working together for your success.
References
Dependability and strong partnerships are our core values, and we are committed to making them a reality. Against this background, we actively involve you in system development, project planning and implementation to ensure that we provide you with exactly what you need: an energy-efficient, resource-saving, highly reliable solution.
